Let The Fireworks Begin... (Page Nine)

The bagpipes call us to the fireworks

Beautiful sound
Did You Know? - Bagpipes are a class of musical instrument, aerophones, using enclosed reeds fed from a constant reservoir of air in the form of a bag. Though the Scottish Great Highland Bagpipe and Irish uilleann pipes have the greatest international visibility, bagpipes of many different types come from different regions throughout Europe, Northern Africa, the Persian Gulf, and the Caucasus. The term is equally correct in the singular or plural, although in the English language, pipers most commonly talk of "pipes."


In The Distance...






Let The Old Ranch Displays Begin...
Did You Know? - America's earliest settlers brought their enthusiasm for fireworks to the United States. Fireworks and black ash were used to celebrate important events long before the American Revolutionary War. The very first celebration of Independence Day was in 1777, six years before Americans knew whether the new nation would survive the war; fireworks were a part of all festivities. In 1789, George Washington's inauguration was also accompanied by a fireworks display. This early fascination with their noise and color continues today.
In 1999, Walt Disney World in Lake Buena Vista, Florida, pioneered the commercial use of aerial fireworks launched with compressed air rather than gunpowder for the Epcot night time spectacular, IllumiNations: Reflections of Earth. The display shell explodes in the air using an electronic timer. The advantages of compressed air launch are a reduction in fumes, and much greater accuracy in height and timing.


Green Barium BaCl2 (barium chloride)





Did You Know? - An aerial firework is normally formed as a shell that consists of four parts:
- Container - Usually pasted paper and string formed into a cylinder
- Stars - Spheres, cubes or cylinders of a sparkler -like composition
- Bursting charge - Firecracker -like charge at the center of the shell
- Fuse - Provides a time delay so the shell explodes at the right altitude
The shell is launched from a mortar. The mortar might be a short, steel pipe with a lifting charge of black powder that explodes in the pipe to launch the shell. When the lifting charge fires to launch the shell, it lights the shell's fuse. The shell's fuse burns while the shell rises to its correct altitude, and then ignites the bursting charge so it explodes.
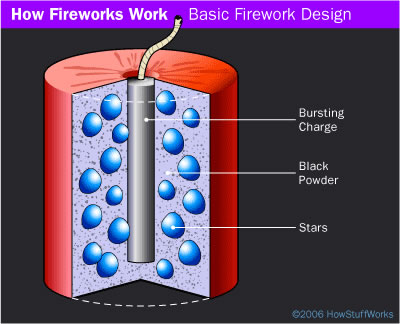
A simple shell used in an aerial fireworks display. The blue balls are the stars, and the gray is black powder.
The powder is packed into the center tube, which is the bursting charge. It is also sprinkled between the stars to help ignite them.
Simple shells consist of a paper tube filled with stars and black powder. Stars come in all shapes and sizes, but you can imagine a simple star as something like sparkler compound formed into a ball the size of a pea or a dime.
The stars are poured into the tube and then surrounded by black powder. When the fuse burns into the shell, it ignites the bursting charge, causing the shell to explode.
The explosion ignites the outside of the stars, which begin to burn with bright showers of sparks. Since the explosion throws the stars in all directions, you get the huge sphere of sparkling light that is so familiar at fireworks displays.
Multibreak Shells
More complicated shells burst in two or three phases. Shells like this are called multibreak shells. They may contain stars of different colors and compositions to create softer or brighter light, more or less sparks, etc. Some shells contain explosives designed to crackle in the sky, or whistles that explode outward with the stars.
Multibreak shells may consist of a shell filled with other shells, or they may have multiple sections without using additional shells. The sections of a multibreak shell are ignited by different fuses. The bursting of one section ignites the next. The shells must be assembled in such a way that each section explodes in sequence to produce a distinct separate effect. The explosives that break the sections apart are called break charges.

Lithium (medium red)
SrCO3 (strontium carbonate)
Li2CO3 (lithium carbonate) LiCl (lithium chloride)


Lithium (medium red)
SrCO3 (strontium carbonate)
Li2CO3 (lithium carbonate) LiCl (lithium chloride)



































































Time to go home.....